Final trial RSV vaccine data released; confirms promising results
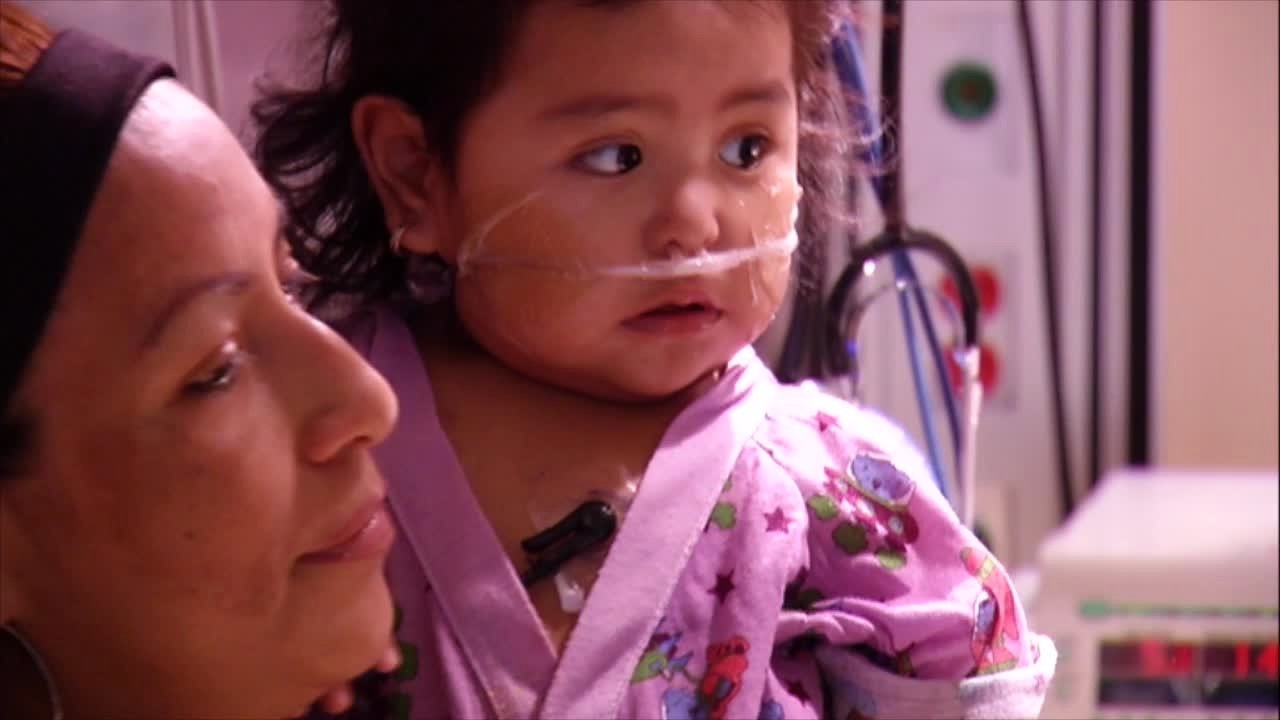
Child in hospital being treated for RSV. (FILE/KSTP)
Final data from a respiratory syncytial virus (RSV) vaccine trial has been released showing a positive efficiency rate at preventing severe infection in infants and confirming the same preliminary data.
Pfizer’s vaccine was given to mothers in the second half of their pregnancy, and vaccine trial results released Wednesday showed the vaccine to be 82% effective in preventing severe infections in infants.
Preliminary results of clinical trials show RSV vaccine protects newborns from severe illness
Allina Health participated in the clinical trial at its site in Coon Rapids, following one pregnant woman from Minnesota and her baby.
Dr. Frank Rhame led the local study.
RSV cases were higher than usual in 2022 for young children and infants, including an early spike, causing hospitals to be overwhelmed at times.
Rhame said he is hopeful it could become widely available before next fall’s RSV season.
RELATED: RSV is filling up Minnesota hospitals, ‘adding up to sort of a perfect storm’
RELATED: Twin Cities hospitals see rise in RSV cases, hospitalizations