Johnson & Johnson among vaccine candidates that could help increase COVID-19 vaccine supply
[anvplayer video=”5002373″ station=”998122″]
This week, the World Health Organization reports there are 63 COVID-19 vaccines in clinical development and another 173 in "pre-clinical development.” The WHO says at least 16 of the COVID-19 vaccines are in Phase III of the clinical trials, which is the final phase.
Some vaccines, like the one being developed by Johnson & Johnson, are eyeing emergency-use approval by the U.S. Food and Drug Administration in the near future – though their efficacy rates haven’t been published yet.
Still, it’s potentially good news for President Joe Biden, who’s held a goal of 100 million people vaccinated in his first 100 days.
"Starting next week… that’s an increase of 1.4 million doses per week,” Biden said on Wednesday.
KSTP’s complete COVID-19 coverage
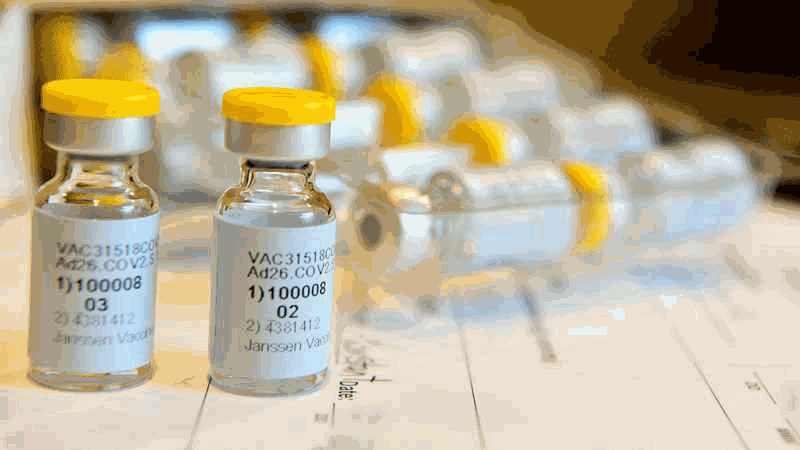
This September 2020 photo provided by Johnson & Johnson shows a single-dose COVID-19 vaccine being developed by the company.[Cheryl Gerber/Courtesy of Johnson & Johnson via AP, File]
“The Johnson & Johnson vaccine, for now, is a single-dose vaccine, whereas the others are a two-dose vaccine. And the other big obvious difference is that the J & J vaccine doesn’t need to be frozen at those artic temperatures,” KSTP Medical Expert Dr. Archelle Georgiou, explained.
That could create some hope, after reports of COVID-19 vaccine distribution shortages throughout the country.
“If the Johnson & Johnson vaccine gets approved, it’s going to be advantageous at a few different levels. One is, we’re going to have one more manufacturer able to make the vaccine, and we all know how much we want more supplies, and they say they’re on track, if the vaccine is approved, to deliver 100 million doses,” Georgiou said. “The other advantage to the Johnson & Johnson vaccine is just that it’s easier to give, it’s easier for a person to get one dose of a vaccine rather than having to go out for a follow-up shot.”
Georgiou also explained that the technology is different.
“The Pfizer and Moderna vaccines are mRNA vaccines that give you this mRNA genetic material that give your body a recipe for making a spike protein, which your body reacts to and creates an antibody. What the Johnson & Johnson vaccine does is that it has altered the DNA of an adenovirus, a non-disease causing virus, that they then give you in the vaccine, and then that virus enters your cells. It’s not able to duplicate, but it is able to stimulate your body through the directions that it gives your own cell to then make an antibody to fight off the COVID-19 vaccine,” Georgiou said.