Medtronic recalls over 348K defibrillators that may not work
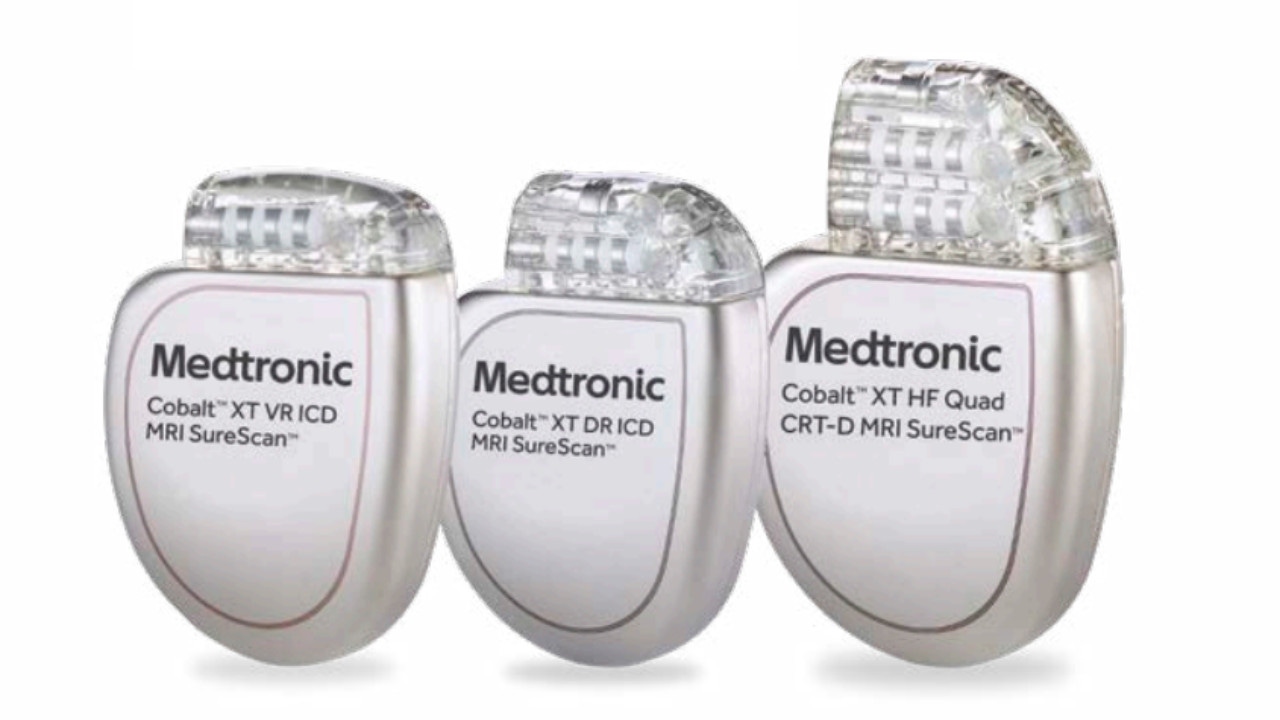
Medtronic's Colbalt XT ICDs and CRT-Ds. (Courtesy: Medtronic)
A Minnesota-based company is recalling more than 348,000 defibrillators over concerns that they may not work.
Medtronic, which has its executive headquarters based in Minneapolis, initiated the recall of implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds) on May 10, according to a notice from the U.S. Food and Drug Administration (FDA).
The recall covers Medtronic’s following products:
- Cobalt XT, Cobalt, Crome ICDs and CRT-Ds;
- Claria MRI, Amplia MRI, Compia MRI, Viva, Brava CRT-Ds;
- Visia AF, Visia AF MRI, Evera, Evera MRI, Primo MRI, Mirro MRI ICDs.
The FDA says the affected devices, which were distributed between October 2017 and June 2023, may have a reduced-energy shock or no shock at all, which could fail to correct life-threatening arrhythmia.
So far, Medtronic has reported 28 incidents and 22 injuries but no deaths from the issue.
Customers with a recalled device are urged to contact Medtronic Technical Services at 1-800-929-4043.
The company released the following statement:
“In May 2023, Medtronic began informing physicians of a potential risk for reduced-energy or no-energy high-voltage therapy in its implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds) manufactured after July 2017. Medtronic provided physicians with comprehensive patient management recommendations in the communication. We have recommended that physicians non-invasively reprogram these devices to reduce the risk for this issue.
The FDA recently designated this voluntary action by Medtronic as a Class I recall. There have been no reports of permanent patient harm or deaths due to this issue in the affected population.”